
Newsletter No1 / July 2024
2) Poster presentations in Trials in Progress category: (i) ESMO Breast 2024 (15 – 17 May) presented by Antonios Valachis (ii) ASCO 2024 (30 May – 3 June) presented by Paris Kosmidis and Thanos Kosmidis
3) A paper based on IMPORTANT project deliverable was recently published: Karihtalaa P, Schiza A, Fountzilas E, Geisler J, Meattini I, Risi E, Biganzoli L, and Valachis A. Clinical trials in older patients with cancer– typical challenges, possible solutions, and a paradigm of study design in breast cancer. Acta Oncologica 2024; 63: 441–447

Newsletter No2 / August 2024
2) The amendment of the IMPORTANT trial protocol regarding some of the inclusion and exclusion criteria is ready for submission (see below in bold)
3) The inclusion criterion on RECIST assessment has been removed 4) The changes in the exclusion and inclusion criteria are to allow a broader patient inclusion and to serve the pragmatic scope of the trial. These changes have no impact on the informed consent form (ICF)

Newsletter No3 / September 2024
2) IMPORTANT trial clinical lead, Antonios Valachis, will be attending the first meeting for Horizon Europe Cancer Mission ‘Diagnosis and Treatment’ cluster in Barcelona, Spain on 12 September 2024. IMPORTANT trial is one of the 12 funded projects under the cluster. Antonis will be chairing the round table discussion on `Addressing Inequalities´

Newsletter No4 / October 2024
2) Clinical sites in Greece have received conditional approval from CTIS
3) The project will request for an extension of inclusion period from 30 months to 36 months, and one year extension
4) Abstract to San Antonio Breast Cancer Symposium (SABCS) 2024 has been accepted for trial in progress poster
5) Prof. Biganzoli presented IMPORTANT as part of her presentation on “MDT– case of an older adult with metastatic breast cancer´´ during ESMO 2024
6) The trial clinical lead, Assoc. Prof. Antonios Valachis, attended the 1st meeting for Horizon Europe Cancer Mission ‘Diagnosis and Treatment’ cluster in Barcelona, Spain on 12 September 2024
7) The project participated at the EU Researcher’s Night event in Nicosia, Cyprus on 27 September 2024, led by Dr Vasiliki Danilatou and Assoc. Prof. Christiana Markou from Eunomia Ltd

Newsletter No5 / November 2024
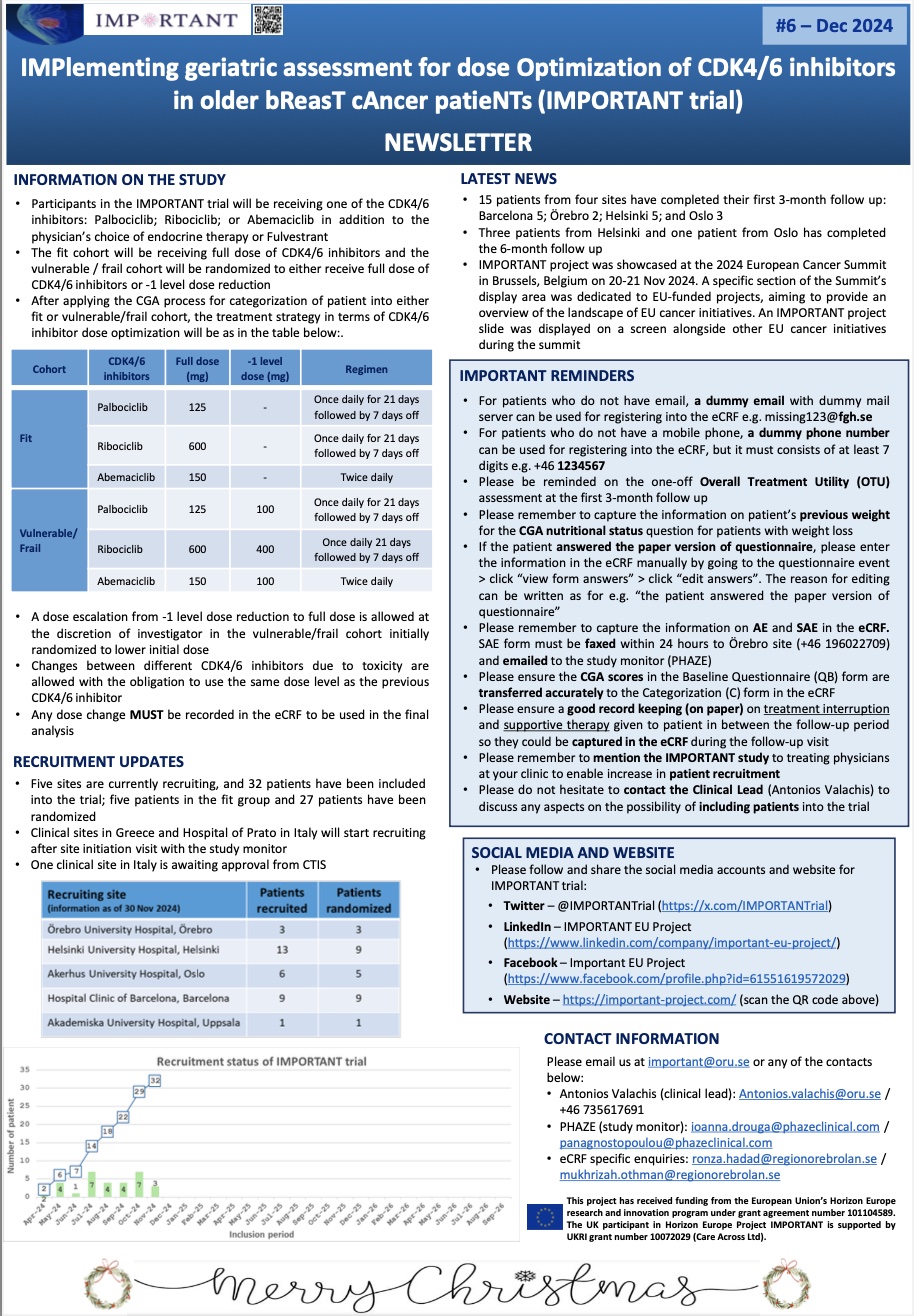
Newsletter No6 / December 2024

Newsletter No7 / January 2025

Newsletter No8 / February 2025
1) Clinical sites in Greece were initiated in January 2025 to include patients
2) St. Luke’s clinic in Thessaloniki, Greece has included one patient
3) A recruitment performance report by HeCOG from April 2024 to January 2025 showed the recruitment progress is currently slightly below target (achieved ~25.3% of 10-month recruitment target)

Newsletter No9 / March 2025
1) The IMPORTANT trial was showcased at the World Cancer Day 2025 project showcase event organized by European Health and Digital Executive Agency (HaDEA) on ‘Fostering synergies to beat cancer: The impact of EU-funded projects’.
2) The event demonstrated the impact of various grants and tenders managed by HaDEA in relation to the Europe’s Beating Cancer Plan and the Cancer Mission

Newsletter No10 / April 2025
1) Hospital of Prato in Italy has included its first two patients
2) Number of patients with completed month follow up in treatment phase: (a) Barcelona 8 (3-month) and 5 (6-month), (b) Helsinki 10 (3-month) and 4 (6-month), (c) Galo 6 (3-month), 3 (6-month) and 2 (9-month), (d) Uppsala 1 (3-month), (d) Orebro 2 (3-month) and 1 (6-month)

Newsletter No11 / May 2025

Newsletter No12 / July 2025
1) IMPORTANT Project met in person on 23 May 2025 for the 4th consortium meeting hosted at the Faculty of Psychology, Universidad Nacional de Educación a Distancia (UNED) in Madrid
2) As of 30 June 2025, 49 patients completed 3-month follow-up, 28 patients completed 6-month follow-up, 16 patients completed 9-month follow-up, and four patients completed 12-month follow-up

Newsletter No13 / August 2025
1) An introduction video to IMPORTANT project has been published and can be watched here: Important Project- V4
(https://lnkd.in/dcrmJ6An)
2) HeCOG has compiled feedback from the patient recruitment survey circulated to IMPORTANT trial clinical sites.

Newsletter No14 / November 2025
1) IMPORTANT project participated at the European Researchers’ Night event in Nicosia, Cyprus on 26 September 2025, led by Dr Vasiliki Danilatou, Assoc. Prof. Christiana Markou from Eunomia Ltd and Kalliopi Mastoraki (CEF)
2) IMPORTANT trial clinical lead, Assoc. Prof. Antonios Valachis, IMPORTANT project coordinator, Helena Hansson- Nylund (ORU), Mukhrizah Othman (ORB), and Kalliopi Mastoraki (CEF) attended the second annual meeting for Horizon Europe Cancer Mission ‘Diagnosis and Treatment’ cluster in Berlin, Germany on 16 October 2025

Newsletter No15 / January 2026
1) Four satellite sites in Sweden have agreed to participate in IMPORTANT trial. All four hospitals are as large as Örebro (Uppsala Hospital is larger than Örebro Hospital):
(a) General Hospital of Vasterås – referral to Örebro (initiation: January
2026)
(b) General Hospital of Gävle – referral to Uppsala (initiation: January 2026)
(c) General Hospital of Karlstad – referral to Örebro (initiation: Jan / Feb 2026)
(d) General Hospital of Eskilstuna – referral to Örebro (initiation: Feb 2026)
