In Brief
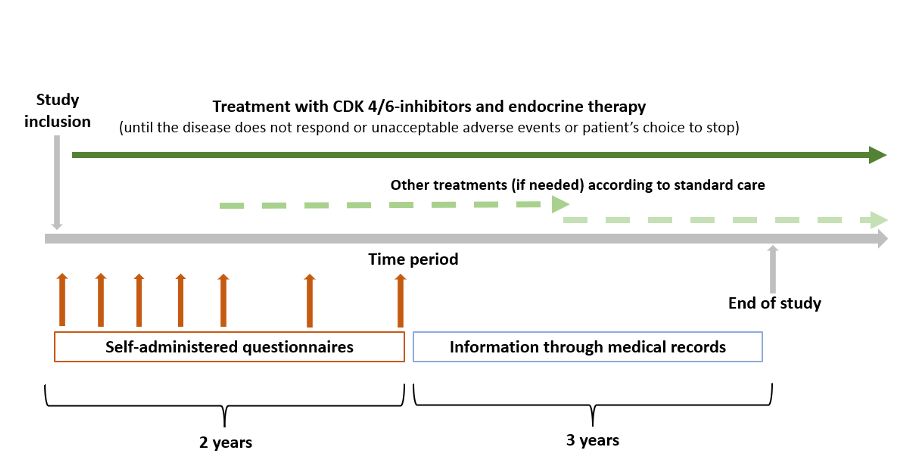
IMPORTANT study is a multi-centre, open-label, prospective, randomized-controlled, non-inferiority trial with a pragmatic approach. This study involves both male and female older patients (≥ 70 years old) with advanced hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer, not amenable for curative treatment and without prior therapy for advanced disease, who are suitable to receive CDK 4/6-inhibitors plus endocrine therapy as first line therapy.
The study implements two approaches with high level of evidence, namely: (1) the use of comprehensive geriatric assessment (CGA) approach in treatment decision making; and (2) the use of CDK 4/6-inhibitors as the initial treatment of choice. The purpose is to investigate whether a common clinical practice (starting dose reduction of CDK 4/6-inhibitors in older patients) with evidence of low certainty can be standardized using a more individualized-based approach.
On the basis of baseline CGA assessment, patients will either receive full dose of CDK 4/6-inhibitors plus endocrine therapy (if patients are fit according to CGA) or be randomized to full dose vs. reduced initial dose of CDK 4/6-inhibitors (if vulnerable or frail according to CGA). The study hypothesis is that adjusting the dose according to vulnerability will allow patients to tolerate treatment better without jeopardizing the treatment efficacy.

Study Population
The IMPORTANT study involves older breast cancer patients (≥ 70 years old) with advanced HR-positive/HER2-negative breast cancer, not amenable for curative treatment and without prior therapy for advanced disease, who are suitable to receive CDK 4/6-inhibitors plus endocrine therapy.

Trial Design
The IMPORTANT study is a phase III & IV Integrated, multi-centre, open-label, prospective, randomized, controlled, non-inferiority trial with a pragmatic approach (Low Intervention Trial)

Aims
- The IMPORTANT study aims to investigate the time to treatment failure in vulnerable or frail older breast cancer patients treated with lower initial dose of CDK 4/6-inhibitors plus endocrine therapy compared to the recommended full dose of CDK 4/6-inhibitors.
Research Questions
Question 1
Question 2
Question 3
Question 4
Does lower initial dose of CDK 4/6-inhibitors provide advantages in terms of patient-reported outcomes?
Question 5
Study Endpoints

Sample Size
30
Months
495
Patients
149
For the fit cohort
346
For the vulnerable / frail cohort
Follow Up